The Carbonado Conundrum: Unearthing the Toughest Diamond with a Cosmic Pedigree
Within the pantheon of precious materials, the diamond is celebrated as the epitome of brilliance, purity, and unparalleled hardness. Yet, beneath the dazzling, flawlessly cut gems used in high jewelry lies a far more mysterious and formidable cousin: the Carbonado diamond. Often referred to commercially as the “black diamond,” the true Carbonado is not just a colored gemstone; it is a geological enigma, a polycrystalline powerhouse whose very existence challenges our understanding of how diamonds are formed.
First identified in Brazil in the 1840s, the name carbonado (derived from the Portuguese word for “burned” or charcoal) perfectly captures its rugged, opaque, and often porous appearance . Unlike the single, large, perfect crystals that define conventional diamonds, the Carbonado is a polycrystalline aggregate—a dense fusion of millions of microscopic diamond crystals cemented together. This chaotic, yet harmonious, internal structure is the key to its unique claim to fame: Carbonados are the toughest form of natural diamond known.
While all diamonds share the maximum hardness of 10 on the Mohs scale, toughness is a different metric, measuring resistance to breaking, chipping, or fracturing. Conventional diamonds have inherent structural weaknesses, or cleavage planes, where a single, well-aimed strike can split the stone. Carbonados, however, due to their random, intergrown crystal orientations, effectively eliminate these planes, granting them superior durability. This makes them profoundly unattractive for fine jewelry—where the term “black diamond” typically refers to treated single-crystal stones—but absolutely indispensable for the most demanding industrial applications, such as heavy-duty drilling and precision cutting.
The properties of the Carbonado—its impurity, its porosity, its lack of association with the deep, volcanic kimberlite pipes that yield all other diamonds—only deepen its mystery. To this day, the definitive birthplace of the Carbonado remains one of the most hotly debated subjects in mineralogy, splitting experts into two dramatically competing camps:
The Terrestrial Subduction Zone Theory: This model posits that Carbonados were forged deep within the Earth, not in the standard mantle but in subduction zones , where ancient, carbon-rich oceanic sediment (laden with organic matter) was dragged far beneath the continental crust. Under immense pressure and temperature, catalyzed by volatile fluids, this organic material crystallized into the unique polycrystalline structure. Proponents of this theory point to the distinct isotopic signature of hydrogen and nitrogen found within Carbonados, which is more characteristic of deep-sea sediments than the Earth’s mantle.
The Extraterrestrial Origin Theory: This much more dramatic hypothesis suggests that Carbonados have a cosmic pedigree. The presence of specific trace elements, particularly the meteoritic mineral osbornite and certain nitrogen and hydrogen isotopes indicative of an interstellar environment, lends credence to the idea that these diamonds were formed in the shockwaves of supernovae explosions or during the catastrophic impacts of celestial bodies, eventually arriving on Earth as fragments of a meteorite.
What is undeniable is the geological scarcity and unique provenance of these gems. Carbonados are only found in alluvial deposits in two regions that were once geographically joined: the Bahia province of Brazil and the Central African Republic (CAR). Recovered from riverbeds and gravels, they are relics of a distant past, estimated to have formed over three billion years ago during the Mesoarchean era—an age comparable to the oldest of Earth’s conventional diamonds.
The Carbonado diamond is thus more than just a stone; it is a profound scientific puzzle locked within a material of unequaled strength. Its continuing mystery not only inspires geologists to look deeper into the Earth (and further into the cosmos) but also underpins the technology that allows us to explore the hardest materials on our own planet. This article delves into the unique chemical, physical, and historical details of this enigmatic aggregate, exploring the theories surrounding its genesis and highlighting why, despite its dark and irregular facade, the Carbonado is truly the king of industrial diamonds.
The Enigmatic Carbonado: Unveiling the Toughest Diamond on Earth

Carbonado Diamonds Origin – beneath the dazzling world of colorless gems lies a hidden realm – the realm of carbonados. Often referred to as “black diamonds,” these unique stones possess a mystique that goes beyond their dark allure.
This article delves into the fascinating world of carbonado diamonds, exploring their unique properties, origins shrouded in mystery, and how they stand apart from their more illustrious crystalline cousins.
What are Carbonado Diamonds?
Unlike the flawless crystals we typically associate with diamonds, carbonados are a far cry from the image of sparkling perfection. These natural diamonds are impure, polycrystalline aggregates, meaning they consist of numerous microscopic diamond crystals fused together. This unique structure gives them a dark, opaque appearance, ranging from black to grey with occasional green or brown hues. Their texture is often irregular and porous, resembling charcoal – hence the name “carbonado,” which translates to “burned” in Portuguese.
Despite their unconventional looks, carbonados boast a remarkable characteristic – extreme toughness. This exceptional property arises from their polycrystalline structure. While they share the same hardness (10 on the Mohs scale) as other diamonds, their multiple crystal orientations create a superior resistance to fracturing. Imagine a single abrasive grain; in a carbonado, it presents various diamond crystal faces, with the strongest orientation handling the cutting action, resulting in longer-lasting and lower-maintenance tools.
The chemical composition of carbonados further sets them apart. They are a fascinating blend of:
- Diamond: The primary constituent, forming the microscopic crystals.
- Graphite: Another form of carbon, contributing to the dark color and porosity.
- Amorphous carbon: A non-crystalline form of carbon.
Additionally, trace elements like nitrogen, hydrogen, and osbornite (a meteorite mineral) have been found in carbonados, hinting at a possible extraterrestrial origin – a theory we’ll explore further on.
Unveiling the Carbonado Diamonds Origin: A Geological Enigma
The journey of a diamond from its formation deep within the Earth’s mantle to our fingertips is well understood. However, the origin story of carbonados remains an enigma. Unlike other diamonds found in igneous kimberlite rocks, Carbonado Diamonds Origin in recovery is primarily discovered in alluvial deposits, formed by the erosion and transportation of weathered rocks. These deposits are often found in mid-elevation equatorial regions, with the most significant discoveries concentrated in:
- Central African Republic – A major source of carbonado diamonds.
- Brazil – Where the first carbonados were identified in the 1840s.
- Venezuela – Another source of these unique black diamonds.
The presence of carbonados in alluvial deposits, coupled with their distinct chemical signature, has led scientists to propose several intriguing theories about their formation:
- Extreme Pressure and Heat: This theory suggests that Carbonado Diamonds Origin formation occurred under immense pressure and high temperatures within the Earth’s mantle, similar to other diamonds. However, the specific conditions required for their unique structure remain unclear.
- Extraterrestrial Origin: The presence of nitrogen, hydrogen, and osbornite in carbonados has fueled speculation about a possible extraterrestrial origin. Some scientists believe they could be remnants of exploded stars (supernovae) or collisions between celestial bodies, eventually reaching Earth through meteorites.
Carbonado Diamonds Origin remains a subject of ongoing research, their unique geological history adds another layer of fascination to these enigmatic gems.
The Mysterious Birthplace: Carbonado Diamonds Origin in Subduction Zones ?
The allure of carbonado diamonds goes beyond their dark beauty. Unlike the well-understood formation process of kimberlite diamonds deep within the Earth’s mantle, the Carbonado Diamonds Origin remains shrouded in mystery. However, a compelling theory suggests their formation could be linked to a dramatic geological phenomenon: subduction zones.
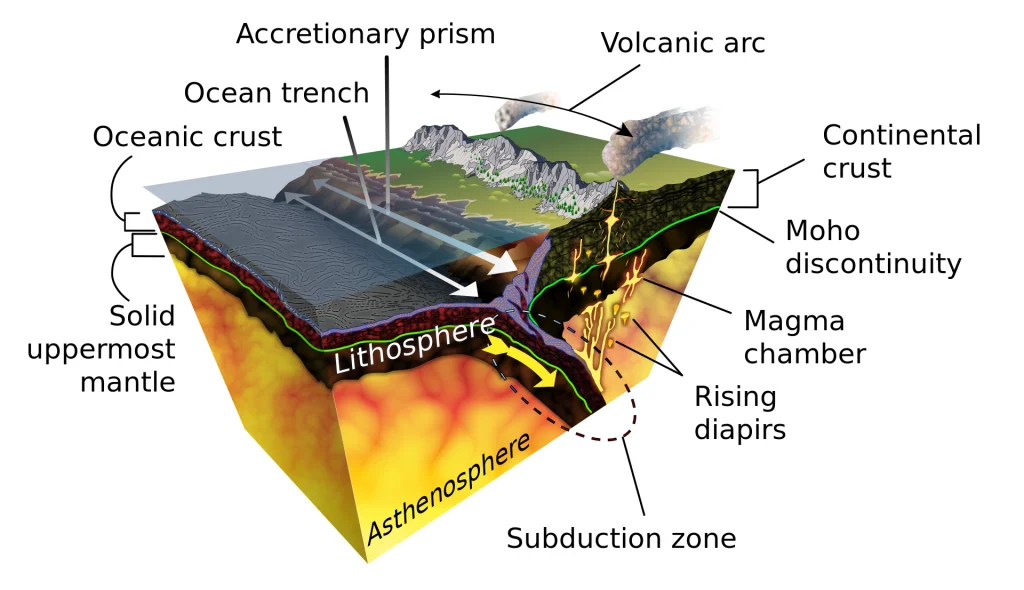
Subduction: A Fiery Underworld Kitchen
Imagine two tectonic plates on a collision course. In subduction zones, one plate, typically the denser oceanic crust, dives beneath the lighter continental plate, plunging into the Earth’s fiery mantle. This descent subjects the subducted plate to immense pressure and scorching temperatures, reaching thousands of degrees Celsius. This intense environment becomes a potential forge for the Carbonado Diamonds Origin.
The Recipe for Carbonado Creation
The exact details of carbonado formation in subduction zones are still being unraveled, but scientists propose a fascinating scenario:
Organic Carbon Feast: The subducting oceanic plate carries various materials, including organic matter from the ocean floor sediments. This organic carbon, rich in elements like hydrogen, nitrogen, and oxygen, becomes a key ingredient in the potential diamond-making process.
Fluid Flux: As the subducted plate descends, it encounters volatile-rich fluids, potentially released from trapped seawater or melted rock. These fluids play a crucial role in transporting and concentrating the essential elements – carbon, hydrogen, and nitrogen – required for diamond formation.
The Pressure Cooker Effect: The immense pressure within the subduction zone, reaching hundreds of thousands of atmospheres, acts as a giant geological pressure cooker. This extreme pressure, combined with the high temperatures, could trigger the transformation of the organic carbon into diamond.
The Impurity Factor: Unlike kimberlite diamonds, which form from pure carbon, the presence of other elements like hydrogen and nitrogen in the subduction zone environment might influence the resulting diamond’s structure. These impurities could be incorporated into the diamond lattice, leading to the polycrystalline and porous nature of carbonados.
The Upward Journey: The newly formed carbonado diamonds might not stay buried forever. Geological processes like volcanic eruptions or tectonic movements can bring these diamonds back towards the surface. They may become trapped in rocks within the Earth’s crust or eventually be eroded and deposited in alluvial deposits, where they are later discovered.
Challenges and Supporting Evidence
While the subduction zone theory presents a compelling explanation for carbonado formation, it faces some challenges:
Temperature Dilemma: Traditional diamond formation requires even higher temperatures than those estimated in subduction zones. Some scientists propose that localized hotspots within the subduction zone could create the necessary temperature conditions.
Diamond vs. Graphite: The high pressures in subduction zones could favor the formation of graphite, another form of carbon, instead of diamonds. Scientists suggest that the presence of specific fluids or unique chemical reactions might tip the scales towards diamond formation.
Despite these challenges, there is some intriguing evidence supporting the subduction zone theory:
Mineral Inclusions: Tiny mineral inclusions, resembling those found in oceanic crust, have been discovered within some carbonados. This suggests a potential link to subducted material.
Hydrogen Signature: The presence of hydrogen isotopes in carbonados aligns more closely with the composition of organic carbon from the ocean floor rather than the Earth’s mantle.
The Evolving Story
The theory of carbonado formation in subduction zones is still under development, with ongoing research aimed at refining the model and gathering further evidence. Experiments simulating the conditions within subduction zones and detailed analysis of carbonado diamonds continue to shed light on their enigmatic origins.
Unveiling the Secrets
The potential link between subduction zones and carbonado diamonds opens up exciting avenues for exploration. Studying these unique diamonds could provide valuable insights into the dynamic processes occurring within Earth’s interior, particularly the behavior of subducted material. Additionally, understanding the formation of carbonados could lead to advancements in creating new diamond-like materials with tailored properties.
The journey to unravel the secrets of carbonado diamonds continues, and the subduction zone theory remains a significant piece of the puzzle. As research progresses, we may one day definitively unlock the fiery birthplace of these remarkable black gems.
Beyond Earth: Unveiling the Origins of Carbonado Diamonds in the Cosmos
 The mystery surrounding the formation of carbonado diamonds extends beyond the confines of our planet. While the subduction zone theory offers a compelling explanation for their Earthly origins, scientists have ventured beyond our home to explore the possibility of a more cosmic birthplace – the violent realm of supernovae and the catastrophic collisions of celestial bodies.
The mystery surrounding the formation of carbonado diamonds extends beyond the confines of our planet. While the subduction zone theory offers a compelling explanation for their Earthly origins, scientists have ventured beyond our home to explore the possibility of a more cosmic birthplace – the violent realm of supernovae and the catastrophic collisions of celestial bodies.
Supernovae: Diamonds Forged in Stellar Explosions
Supernovae, the spectacular explosions of massive stars, are some of the most energetic events in the universe. These colossal blasts not only scatter the star’s material across vast distances but also create the conditions necessary for forging a variety of elements, potentially including the unique carbon structures found in carbonado diamonds.
The Diamond Factory in a Supernova:
Stellar Crucible: As a massive star nears the end of its life, its core runs out of fuel to sustain nuclear fusion. This imbalance triggers a dramatic collapse, leading to a surge in temperature and pressure. The core conditions become ripe for the creation of new elements through a process called nucleosynthesis.
Diamond Rain: Within the collapsing core, immense pressure could squeeze carbon atoms together, potentially forming diamond crystals. However, the extreme environment within a supernova favors the formation of smaller diamond structures called nanodiamonds. These microscopic diamonds, typically less than 100 nanometers in size, could be the building blocks of carbonados.
The Great Escape: The supernova explosion violently ejects the newly formed elements, including nanodiamonds, into the interstellar medium. These diamonds become interstellar wanderers, traveling for millions or even billions of years.
Cosmic Collisions and Planetary Birth: The ejected nanodiamonds could eventually become part of the swirling dust and gas clouds that give birth to new stars and planetary systems. During the formation of planets, these nanodiamonds might become incorporated into the protoplanetary disk, the swirling material that surrounds a young star.
Planetary Pressures: Within the protoplanetary disk, the nanodiamonds could be subjected to intense heat and pressure as the protoplanet coalesces. This extreme environment might cause the nanodiamonds to fuse together, forming the larger polycrystalline aggregates that characterize carbonado diamonds.
Challenges and Supporting Evidence
The theory of carbonado formation in supernovae presents some intriguing possibilities, but it also faces significant challenges:
Survival of the Smallest: Nanodiamonds are incredibly small and delicate. It’s unclear how they would survive the violent environment of a supernova explosion and the harsh journey through interstellar space.
Planetary Incorporation: The process of incorporating nanodiamonds into a forming planet and subjecting them to the necessary pressure and heat for further growth remains unclear.
Despite these challenges, some evidence lends credence to the possibility of a cosmic origin for carbonados:
Isotopic Signature: Analysis of the nitrogen isotopes found in some carbonados suggests a possible non-terrestrial origin, potentially aligning with the composition of nitrogen found in certain stellar environments.
Osbornite Inclusion: The presence of osbornite, a rare mineral typically found in meteorites, within some carbonados hints at a potential extraterrestrial connection.
Planetary Collisions: A Celestial Diamond Forge
Another cosmic scenario proposes that carbonado diamonds could form during the catastrophic collisions of celestial bodies like asteroids or comets. The immense forces unleashed during such impacts could replicate the extreme pressure and heat conditions believed to be necessary for diamond formation.
The Impact Forge:
Celestial Crash: A colossal collision between two celestial bodies generates tremendous pressure and heat at the point of impact. This intense environment could trigger the transformation of any existing carbon-rich material, like organic molecules within asteroids or comets, into diamond structures.
Diamond Shrapnel: The impact shatters the colliding bodies, potentially ejecting newly formed diamond fragments, including carbonado-like structures, into space.
Earthly Arrival: These diamond-bearing fragments could eventually reach Earth through meteorite impacts, depositing carbonados on our planet’s surface.
Challenges and Supporting Evidence
Similar to the supernova theory, the collision theory also faces some hurdles:
Recreating the Conditions: Simulating the exact pressure and temperature conditions necessary for diamond formation during an impact event remains a challenge.
Identifying Collision Diamonds: Distinguishing between carbonados formed through impacts and those with a terrestrial origin requires further research and analysis techniques.
Despite these challenges, some evidence supports the possibility of a collisional origin for carbonados:
Irregular Shapes: The irregular and fractured nature of some carbonados could be a result of the violent forces experienced during a celestial collision.
Distribution Patterns: The occasional discovery of large clusters of carbonado diamonds might suggest a common origin, potentially from the breakup of a larger diamond.
Beyond Black: The Unique Properties of Carbonado Diamonds
While not used in jewelry due to their opaque nature, carbonado diamonds possess properties that make them invaluable for industrial applications. Here’s what makes them stand out:
- Unequaled Toughness: As discussed earlier, their polycrystalline structure grants them exceptional resistance to wear and tear.
- High Thermal Conductivity: Carbonados efficiently conduct heat, making them ideal for applications involving high temperatures.
- Chemical Inertness: They exhibit minimal reactivity with most chemicals, allowing for their use in harsh environments.
These properties make carbonado diamonds the go-to material for a variety of industrial applications, including:
- Cutting and Grinding Tools: Their incredible cutting ability makes them perfect for sawing, drilling, and polishing hard materials like stone, concrete, and asphalt.
- Oil and Gas Exploration: Their resistance to wear and tear makes them crucial for downhole drilling equipment used in extreme conditions.
- Microwaves and Electronics: Their thermal conductivity properties find use in heat sinks for electronic components.
- Advanced Materials Research: Carbonado diamonds are being explored for their potential use in creating new superhard materials.
Here are 10 frequently asked questions about the enigmatic Carbonado, or “black diamond,” covering its unique properties, mysterious origin, and practical use.
1. What exactly are Carbonado diamonds, and how do they differ from regular diamonds?
Carbonados are a unique form of natural diamond known as polycrystalline aggregates (or diamondites). Unlike traditional diamonds, which are single, flawless crystals, carbonados are composed of millions of microscopic diamond crystals fused together. This structure makes them opaque (black, grey, or dark brown), often porous, and gives them a texture resembling charcoal.
2. Why are Carbonado diamonds considered the “toughest” type of diamond?
Carbonado diamonds share the same hardness (10 on the Mohs scale) as other diamonds, but their unique polycrystalline structure makes them significantly tougher. Toughness is resistance to breaking, chipping, or fracturing. Because carbonados are an aggregate of many crystals oriented randomly, they lack the clear cleavage planes found in single-crystal diamonds, giving them superior durability for industrial applications.
3. What are the main theories about the mysterious origin of Carbonado diamonds?
There are two primary, competing theories regarding the origin of Carbonados, which remains a geological enigma:
Extraterrestrial Origin (Cosmic): This theory suggests Carbonados formed in outer space, possibly in supernovae explosions, and arrived on Earth via meteorites. Evidence includes the presence of specific trace elements like hydrogen and nitrogen isotopes, and the mineral osbornite (a meteorite mineral), found within some carbonados.
Terrestrial Subduction Zone Origin: This theory proposes they formed under immense pressure and heat deep within Earth’s crust in subduction zones, where organic, carbon-rich materials from the ocean floor were dragged into the mantle.
4. What is the chemical composition of a Carbonado diamond?
Carbonados are impure forms of diamond. Their composition is primarily carbon crystallized as diamond, but they also contain significant inclusions of graphite and amorphous carbon, which contribute to their dark color and porosity. Trace elements like nitrogen, hydrogen, and osbornite are also frequently present.
5. Where in the world are Carbonado diamonds found?
Carbonado diamonds are extremely rare and have only been definitively found in alluvial deposits in two primary locations that were once geographically connected (part of the ancient supercontinent Rodinia):
Brazil (Bahia Province)
Central African Republic (CAR) They have also been reported in Venezuela. Unlike conventional diamonds, they are never found in kimberlite pipes (volcanic rock from the mantle).
6. Are Carbonado diamonds used in jewelry?
Generally no, Carbonado diamonds are not preferred for traditional fine jewelry due to their opaque, irregular, and porous nature. The term “black diamond” is often used to market heavily treated or colored single-crystal diamonds in the jewelry trade. The true Carbonado is overwhelmingly used for its superior industrial properties.
7. What are the main industrial applications of Carbonado diamonds?
Carbonado’s unequaled toughness, combined with its high thermal conductivity and chemical inertness, makes it invaluable for demanding industrial applications. These include:
Cutting and Grinding Tools: Used for sawing, drilling, and polishing extremely hard materials like rock, concrete, and asphalt.
Drill Bits: Crucial for downhole drilling equipment in oil and gas exploration due to their wear resistance.
Precision Abrasives: Used in applications requiring superior longevity and cutting effectiveness.
8. Why do the theories about Carbonado’s origin involve hydrogen and nitrogen?
The presence of specific hydrogen and nitrogen isotopes within Carbonados provides crucial clues. Scientists believe these elements are characteristic of either:
Interstellar space or the atmosphere of early, hydrogen-rich celestial bodies (supporting the extraterrestrial theory).
Organic carbon-rich matter found in deep-sea sediments, which would be carried into subduction zones (supporting the terrestrial theory).
9. Why are Carbonados also called “black diamonds”?
The name “black diamond” is a common, often commercial, term for the dark, opaque stones. The original name, “carbonado,” was coined by the Portuguese in Brazil in the 1840s, derived from its visual resemblance to charcoal (“burned” in Portuguese).
10. Are Carbonado diamonds older than regular diamonds?
The Carbonado diamonds recovered are generally estimated to have formed about 3 billion years ago (Mesoarchean era). This age is comparable to some of the Earth’s oldest conventional diamonds, but the key difference is where and how they formed, which is still being actively researched.
[Contact Andy Turkington] | [View the 2026 Tour Itinerary] | [Shop the Collection]
